Bioelectric stimulation designed to reverse remodel failing hearts!
Bioelectric stimulation sequence to not only stave off fibrosis but reverse it converting previous fibrotic scar tissue to new beating pumping muscle! LEARN MOREBioelectric stimulation designed to reverse remodel failing hearts!
Bioelectric stimulation sequence to not only stave off fibrosis but reverse it converting previous fibrotic scar tissue to new beating pumping muscle! LEARN MOREPatented bioelectric signal sequence for homing, proliferating and differentiation of stem cells into new beating, pumping heart muscle, even in the depths of myocardial scar tissue!
Patent pending bioelectric signal sequence for real time reading, managing and modulation of inflammation. A key to managing and recovering from advanced heart failure! Our team believes that the only way to manage inflammation properly is through real time reading and real time bioelectric signal delivery for the controlled release of inflammation management proteins!
Patent pending bioelectric signal sequence for follistatin expression the most powerful protein known to help muscle regeneration and recovery!
Patent pending bioelectric signal sequence for tropoelastin expression for increasing elasticity of heart muscle, the aorta, and arteries!
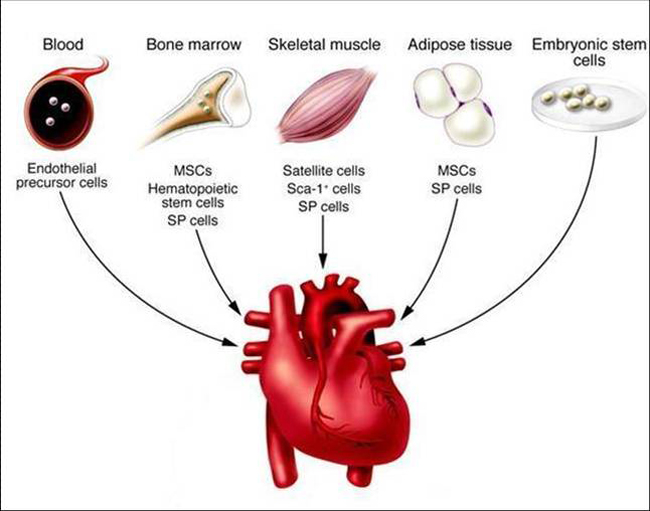
Unmatched research team experience! We completed our first muscle stem cell heart regeneration studies in large animals in 1988 and published our first bioelectric regeneration paper in CIRCULATION the Journal of the American Heart Association in 1999! Our team led a group that completed the historic landmark first ever non-surgical muscle stem cell repair of a human heart in 2001 published in the Journal of American College of Cardiology in 2003!
1. Repeat daily, weekly or monthly slow micro pump infusion deliveries of not only muscle stem cells but a full composition of support factors such as growth factors, exosomes, alkaloids, micro RNAs, nutrient hydrogel, follistatin, SDF-1 and matrix. Management of inflammation, cell retention, survival, proliferation and differential is essential for success!
2. Bioelectric stimulation of heart regeneration promoting proteins (19+) + bioelectric real time inflammation management.


Leonhardt MicroStimulator II
Leonhardt Implantable Micro Stimulator Option
BioLeonhardt is a world leader in developing innovative heart regeneration therapies
The Company has established what it believes is the industry’s most comprehensive and advanced technology platform portfolio for total heart regeneration.
Our platform technology components include:
- Bioelectric stimulator, signals and sequences for precise control of regeneration promoting proteins and for managing inflammation.
- Micro infusion pump for precise slow infusion delivery of our BL-15 mixed stem cell based heart regeneration composition.
- BL-15 fifteen component heart regeneration composition.
(1) Bioelectric Stimulation for heart regeneration.
- Quarter size micro implant stimulator – implanted in chest or abdomen like a pacemaker under the skin.
- Bioelectric heart regeneration program can be loaded into standard CRT/ICD pacers if desired.
- One lead to center of myocardial scar for 1st. generation delivery. Future is non-invasive with temporal interference technologies.
- Patented stem cell homing signal – SDF-1+.
- Patented stem cell differentiation into heart muscle signal within myocardial scar tissue.
- Patented combination of bioelectric stimulation and mixed stem cell based composition for heart regeneration.
- Patent pending follistatin expression bioelectric signal sequence for muscle regeneration.
- Patented VEGF bioelectric signal sequence for new blood vessel growth.
- Patent pending angiogenic bioelectric signal sequence for mature new blood vessel growth VEGF, PDGF, IGF1, EGF, HGF, Tropoelastin, HIF1a, CXCL5, eNOS and SDF-1.
- Patent pending DNA and cell repair protein expressions.
- Patent pending real time inflammation reading and management sequence.
- CXCL5 controlled release for preventing arteriosclerosis following heart regeneration.
- Sequence program patent pending for pre-treating scar, treating scar and post treatment maintenance all repeated until total heart regeneration is achieved.
- Bioelectric signal sequence for managing blood pressure following heart regeneration.
(2) Micro infusion pump
- Tested and developed over the last 5 years with over $2 million in NSF Small Business Innovation Research grant support.
(3) BL-15 Mixed stem cell based mixed composition for heart regeneration.
- Stem cells.
- Selected exosomes.
- Amniotic fluid (chock full of over 200 growth factors)
- Selected Micro RNAs.
- Selected alkaloids such as tetraharmine.
- PRF – platelet rich fibrin (chock full of slow release growth factor).
- Nutrient engineered hydrogel.
- Follistatin.
- Tropoelastin in hydrogel.
- Heart matrix.


Combination micro bioelectric stimulator and re-fillable infusion pump
We believe heart regeneration results in advanced heart failure patients can be improved even more with the addition of our Second Heart Assist, Inc. www.secondheartinc.com chronic wireless powered implant circulatory assist pump within an aortic stent placed just above the renal arteries. This can serve to reduce fluid overload, reduce heart workload, improve perfusion and multiple organ health during the up to 36 month course expected for total heart regeneration.
Warning: Investigational Use Only. Not yet proven safe or effective in controlled clinical trials.
